

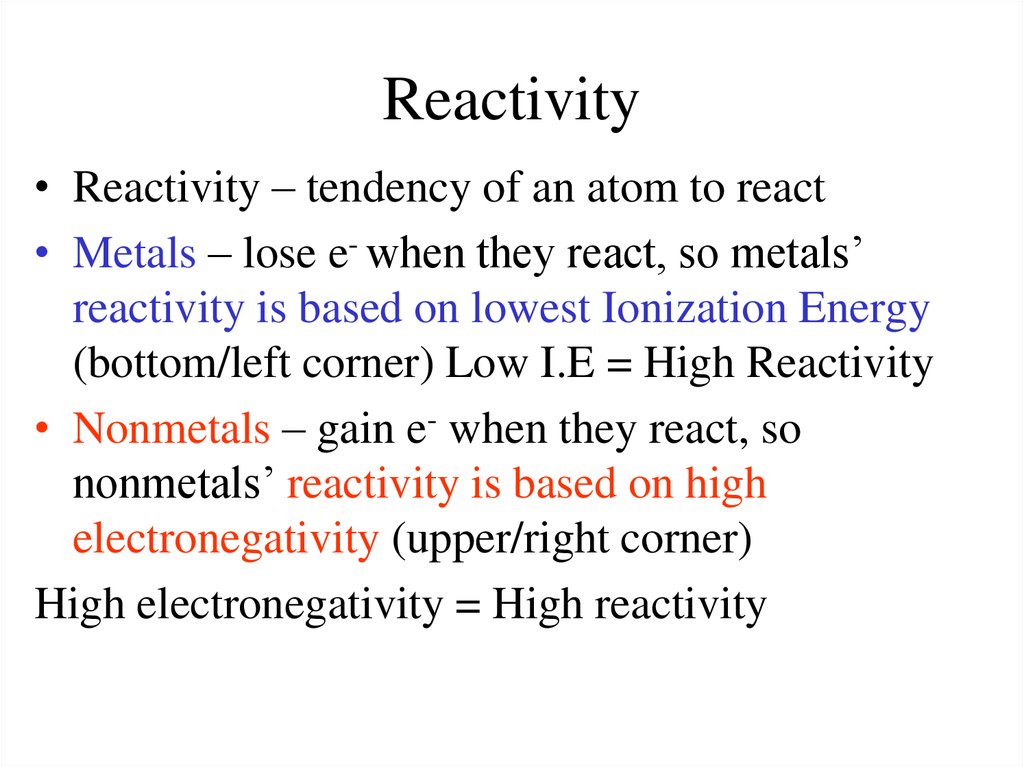
The occurrence of multiple oxidation states separated by a single electron causes many, if not most, compounds of the transition metals to be paramagnetic, with one to five unpaired electrons. For reactor core with eff 0.006 (0.6), one dollar is equal to about 600 pcm. The most common units for power reactors are units of pcm or K/K. Mathematically, reactivity is a dimensionless number, but various units can express it. Because of the slow but steady increase in ionization potentials across a row, high oxidation states become progressively less stable for the elements on the right side of the d block. The reactivity may be used as a measure of a reactor’s relative departure from criticality. Manganese, for example, forms compounds in every oxidation state between −3 and +7. The relatively small increase in successive ionization energies causes most of the transition metals to exhibit multiple oxidation states separated by a single electron. Thus all the first-row transition metals except Sc form stable compounds that contain the 2+ ion, and, due to the small difference between the second and third ionization energies for these elements, all except Zn also form stable compounds that contain the 3+ ion.
This in turn results in extensive horizontal similarities in chemistry, which are most noticeable for the first-row transition metals and for the lanthanides and actinides.

The similarity in ionization energies and the relatively small increase in successive ionization energies lead to the formation of metal ions with the same charge for many of the transition metals. Trends in Transition Metal Oxidation States As a result, the metals in the lower right corner of the d block are so unreactive that they are often called the “noble metals.” The electronegativity of the elements increases, and the hydration energies of the metal cations decrease in magnitude from left to right and from top to bottom of the d block. \): Some Trends in Properties of the Transition Metals.


 0 kommentar(er)
0 kommentar(er)
